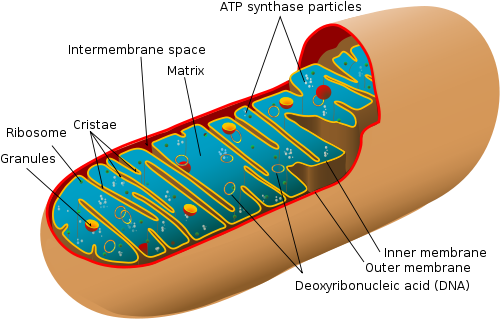
A fascinating study [1] has recently underscored the hypothesis that our mitochondria, the power packs of our cells, share an ancient kinship with a particularly nasty disease, typhus. Put plainly, the same bacterium may have been the granddaddy of each. This result surprisingly implies that we’re all partly bacterial, and not only in a genetic sense but also structurally. In the last post (“Frankenstein’s Batteries“), I covered the story of how a forced cohabitation with our one-celled ancestors could have made the originally disease-causing bacterium change its stripes and give rise to the essential mitochondria that now power our cells. Dedicated components of our cells (organelles) have been acquired via the process of endosymbiosis, in which one organism lives within the body of another in order to gain access to benefits such as a reliable source of nutrients or protection from the elements.
But many people, especially if they have been courted by the ideology of creationism or intelligent design, might respond: “Fascinating story, but too bad you don’t have evidence of intermediate stages of the process. All you have are fully parasitic microbes and fully integrated organelles, right? Can you point to any living candidates of in-between stages?” To this question, an over-enthusiastic biologist — not unlike myself — would bristle with excitement and answer: “Glad you asked! Let’s rectify this problem and fill the alleged gaps with some colorful living examples.”
So let’s look at some weird and wonderful examples of living intermediate stages in the taming of another organelle, the chloroplast. But first, let’s consider how chloroplasts appear to have evolved, and the evidence for their evolutionary origin.

Cells of the moss Plagiomnium affine, showing the numerous brilliant green disk-shaped chloroplasts inside each cell. (Image: Kristian Peters -- Fabelfroh)
Plants and Algae: Hunters-Turned-Farmers
Plant and algae cells have mitochondria, the same as we do. (Recall, from the last post, that mitochondria are the power plants in our cells, churning out useful energy by ‘burning’ the food molecules that we ingest.) But the ancestors of plants had an even better idea than did our animal cells’ ancestors. (Of course, you need to read “idea” in a figurative sense only — cells have no conscious volition or thought processes.) Plants’ ancestors acquired an improved version of an energy generating device — a chloroplast — by engulfing cyanobacteria, a photosynthetic group of bacteria that are also known as “blue-green algae”. The chloroplast differs from the mitochondrion in that it appears to have arisen not from the invasion of a host cell by a parasite, but from a predator playing with its food before digesting it. Nevertheless, it’s a splendid example of endosymbiosis.
In those early days, plant cells’ ancestors did not possess the green color that they do today. Whatever green hues they did sport probably came from the cyanobacteria that they ate, and it lasted for the short time before they proceeded to digest the cells, pigments and all. Cyanobacteria are good sources of food because they are full of chemical energy. Cyanobacteria use the green pigment chlorophyll (in collaboration with red, orange and yellow pigments called carotenoids) to convert, or transduce, solar energy from the sun into useful chemical energy. They do this by cocking the hammer, as it were, on high-powered molecules such as adenosine triphosphate, or ATP. But they also store some of the energy from light in the energetic chemical bonds within sugar molecules. This way, they not only get abundant free energy from the sun, but are also able to shelf some of it in a stable form that their mitochondria convert into ATP later at night, when the sun is not around. Their solar-powered habit made cyanobacteria palatable to other types of cells in their environment — predatory cells. These predators feasted on cyanobacteria for countless millions of years. But then a revolutionary event occurred. It may have gone something like this:
A predatory eukaryotic cell slurped up cyanobacteria from the warm surface of a pond, lake or ocean, engulfing them by phagocytosis — i.e. wrapping a membrane around them and then pumping digestive enzymes into the membrane to dismantle the cyanobacteria. The simple digested molecules were then fed into mitochondria, which turned them into energy (ATP) for the cell. But the cyanobacteria continued to make sugar by photosynthesis for a short time before they were digested in the phagocytosis membrane. Some of this sugar leaked out into the surrounding cell, and for a short time before digestion, the predatory cell enjoyed photosynthetically-manufactured food, courtesy of the cyanobacterium’s last gasps. Some cells refrained from digesting their captured cyanobacteria because it turns out that if they stockpiled a whole lot of these membrane-coated cyanobacterial ‘candies’ and let them continue to live while restraining their rate of reproduction, then the predators (now hosts) could soak up so much sugar from their cyanobacterial captives that they didn’t even need to expend energy to ingest more cyanobacteria. They could just bake in the sun all day long and let their cyanobacterial vending machines pump out sugar for their mitochondria to convert into energy.
And so the chloroplast was born, a membrane-enclosed organelle in plant and algal cells that carries out photosynthesis. Of course, cells did not make any conscious decisions to refrain from digesting cyanobacteria. But when several ways of life are available to an organism, the more economically prudent option (with a high ratio of benefit to cost) results in more offspring, making this way of life ever more prevalent in the population if it can be inherited genetically. Retention of living cyanobacteria offered the great opportunity to abandon the expensive business of hunting at the small cost of foregoing the energy from digesting the whole cyanobacterial cell. It was a little like the transition that our own human ancestors made thousands of years ago: farming presented a good return on the investment of giving up hunting.

Cross-section of a generic chloroplast, the organelle in which photosynthesis occurs in plants and algae. Its structure and genome is very similar to that of ancestral cyanobacteria. (Image: Miguelsierra)
Evidence for Cyanobacterial Ancestry of Chloroplasts
As for mitochondria, there’s evidence of a bacterial origin for chloroplasts. Like mitochondria, chloroplasts possess their own DNA (called the plastome), which is a circular loop, more like that in bacteria than in the eukaryotic host cell. This DNA is severely reduced, containing only about 5% as many genes as free-living cyanobacteria, but the genes that are present are similar to those of their cyanobacterial ancestors. And the missing DNA? Amazingly, much of it has been transferred from the chloroplast to the host’s own chromosomes in the nucleus. This extensive genetic cross-wiring illustrates that stable endosymbiosis has caused an impressive amount of the labour of living to be divided between the host and its photosynthetic guest.
Farming photosynthetic organisms by engulfing them and then holding them captive to soak up their sugary cell products is such a good strategy for survival that there is evidence that it has taken place multiple times in the past in different groups of cells. Molecular biologists can recognize at least two genetic lines of chloroplasts, meaning that cyanobacteria were engulfed and successfully ‘domesticated’ on at least two separate occasions (in the Archaeoplastida, which includes plants and algae on the one hand, and in the amoeboid cell Paulinella on the other hand). Furthermore, within the main Archaeoplastida genetic line, we see evidence of not only primary endosymbiosis (establishing chloroplasts by engulfing naked cyanobacteria), but also secondary endosymbiosis. This means that some algae, such as Vaucheria, appear to have engulfed an algae that had in turn engulfed a cyanobacterium ages ago. The whole arrangement now resembles Russian nesting dolls, with the original cyanobacterial descendent wrapped in four concentric membranes corresponding to the two membranes of the chloroplast nested within the cell membrane of its primary host and wrapped in the feeding membrane of its secondary host. In some cases (e.g. cryptomonads), the original nucleus of the engulfed algal cell is still found squashed between the membranes of the chloroplast.

An evolutionary tree showing the relatedness of different lineages of chloroplasts in living groups of organisms. Generally, each major lineage originates from a unique event of endosymbiosis, the establishment of a stable association between a host and either a cyanobacterium or an algae that already harbours a chloroplast. (Image: Nova)
Almost a Chloroplast: Intermediate Stages in Evolution
But there’s a catch: if this predator-gone-plant way of life was so attractive that it arose more than once, then we should expect to see it happening today as well. We should see some intermediate stage of chloroplast taming going on. Evolution doesn’t normally just work once and then stop. Predatory cells that feed on photosynthetic cells should still be embarking on similar collaborative business ventures with their food. So where are they? They’re all around us, it turns out.
A Fungus Took a ‘Lichen’ to Some Algae…
Lichens are a weird chimaeric life form with an almost absurdly robust ability to survive under extreme conditions, such as the soil-free surface of wind-lashed rocks on the peaks of mountains, tomb stones, tree trunks, old rusting automobiles, soil, sand, etc. They derive their versatility partly from their compound nature: lichens consist of a durable fungus (normally a heterotroph, which gets its food from digesting other organisms) whose internal cells are modified into finger-like formations that clasp single cells of cyanobacteria or green algae (like a long-fingered basketball player holding a ball between dribbles), and forcing the membranes of the algae to leak out some of the sugars that it produces. The lichen is thus effectively a photosynthetic fungus, powered by the sun.

A lichen called Xanthoparmelia growing on basalt, a volcanic rock, on O'ahu, Hawaii. The shape of the creature is determined by the fungus, and the subtle green color is due to algae held captive under the upper cortex (skin-like layer) of the fungus. The color is only faintly green because light does not penetrate as easily through the lichen's cortex as it does through the cells of a plant leaf, so most of the color is due to the fungal portion of the creature. Lichens are more obviously green when they are wet, partly because their tissues are more transmissive to light under those conditions. (Image: Eric Guinther)
Lichens are interesting in that the algae do not grow inside the fungus cells (unlike a plant, which has cell-bound chloroplasts), but are held within the body of the many-celled fungus nonetheless. The relatively primitive nature of the association is also underscored by the fact that the algal or cyanobacterial cells have to be captured from the environment each time that the fungus portion reproduces from spores. However, it may be hard to find the algae that it needs to survive in some of the wretched environments in which it lives, and the lichen may die out if it needed to catch its food-producing algae each time it reproduced. So the lichen solves the problem by also being able to reproduce by fragmentation, and by releasing little balls of fungal cells that are already clasping algal cells.
Corals and Anemones: Alternative Green Energy on the Reef
Animals are usually pretty good at getting around and hunting for their food. But say you’re an animal without legs or a brain — not unlike a coral or sea anemone in the ocean. This predicament makes hunting for your food pretty difficult, and you’re forced to rely on laying in wait for snacks to come bumbling along and to get close enough for you to ensnare them in your tentacles. So you’d better hope (also a trifle difficult without a brain) that you live in a heavily populated lagoon, with lots of “accidental” feeding opportunities. It wouldn’t be a bad idea to have a backup plan in case your neighborhood experienced a drop in property value and stopped attracting tourists.

Two examples of mutualism, an interaction between species that mutually benefits each. In the first example, the clownfish hides from its enemies among the poisonous tentacles of the anemone (to which it has evolved a defense) while the anemone derives both nutrients from the fish's wastes and protection against anemone-eating butterfly fish (which the clownfish drives off). In the second example, the tentacles of the anemone are green because of the endosymbiotic algae that live in its cells, providing the host cell with photosynthetically produced sugars in exchange for a stable habitat in which to live, free of grazing. In effect, the algae serve the animal in the same way that chloroplasts serve a plant, and only part of an anemone's food consists of other animals that it consumes. This is why bleaching of corals (the loss of the endosymbiotic algae) is so damaging to reefs; it starves them of solar-generated food. (Image: Janderk)
In an energy crisis, going green is advisable. Unlike a great deal of conservative politicians, many comparatively highly evolved corals and anemones have embraced precisely this strategy. They have solved the compound problem of an unpredictable food supply and a sessile lifestyle by hosting algae within their own cells and harvesting the chemical energy that their windowsill gardens provide. The bizarre result is an effectively photosynthetic animal. If you’ve ever looked into an aquarium and seen a green colored coral, or an anemone waving verdant tentacles, it’s the endosymbiotic algae that are responsible for this hue.

Coral polyps (the animal units of colonies that form the large corals with which we are familiar), tinged green by their dinoflagellate algal endosymbionts. (Image: Nbarakey)
Endosymbiotic algae in corals much more closely resemble cloroplasts than do the algae in lichens. Not only do they reside within cells than between them, but they can also be transmitted to the next coral generation in egg cells, circumventing the need for the animal to acquire its algal cells from its environment. But although the algal cells are more integrated into the coral’s system than those found in lichens, they are still not quite chloroplasts. The association between corals and algae is not as tightly coevolved as the plant/chloroplast association, in which many of the genes for controlling reproduction have been surrendered by the cyanobacterium (now a chloroplast) to the plant’s chromosomes. The algae can survive perfectly well independently of the coral (perhaps even better, leading to the suggestion that the coral actually parasitizes the algae), and corals can both collect populations of algae from their environment and release algae from their cells.
Algal cells are prevented from multiplying out of control and bursting the cell like a virus by (1) digestion of excess cells, (2) production of chemicals that prevent the algal cells from multiplying, or (3) the above-mentioned release of algae from coral cells [2]. In fact, it’s a malfunction of this ability of corals to release their endosymbiotic algae that leads to the serious problem of coral bleaching on reefs. An upset of their environment by factors such as warm ocean temperatures can cause the release of so many algal cells that the corals starve.

Coral bleaching due to the expulsion of endosymbiotic algae. Normal coral on the right, its greenish hue due to the algae in its cells; bleached coral on the right after ejecting its complement of photosynthetic cells. (Image: NOAA)
The Disposable Solar Batteries of Dinoflagellates
Many other types of life forms also acquire algae from their environment and use them as power sources. They include not just animals, but also single-celled predators. Dinoflagellates are one such group of unicells, oddly shaped creatures that look like plated military helmets plastered together. Not all species of dinoflagellates harbour endosymbiotic algae, and even those that do can expel them and shift back to a predatory lifestyle. In this sense, dinoflagellates use algae like disposable batteries, and the impermanent nature of the interaction underscores the incompleteness of evolution toward a stable association. In a bizarre twist of fate, dinoflagellates are sometimes also enslaved as endosymbionts by certain corals.

A typical dinoflagellate cell (Peridiniella danica), which is oddly shaped due to its cell wall forming grooves along which lie their two characteristic whiplike flagella, with which they propel themselves through the water. (Image: Crassiopeia)
Hatena arenicola: The Jekyll and Hyde Microbe
Among the tiny single-celled creatures that keep captive algae to freeload on their photosynthetic products is an odd creature called Hatena, a scientific name which means “enigma” in Japanese — an apt name [3]. It represents one of the best examples of an association that is transitional between free-living species and an obligately joined union, because of several modifications to each partner that facilitate their association.
Each cell of Hatena harbours only a single large endosymbiotic cell of the algae called Nephroselmis. This algae possesses its own dedicated chloroplast (so recall that we’re dealing with secondary endosymbiosis here), but once the Hatena acquires the algae, the chloroplast is severely enlarged, and other cell components, such as mitochondria, Golgi body, cytoskeleton, and the internal membrane system are degraded. These are major modifications that maximize the efficiency of the algae as a solar-energy converter, reducing those parts that it would only need when living on its own.
Oddly, when Hatena divides, one daughter cell keeps the algal cell and remains photosynthetic, living a life like a plant, while the other daughter cell turns predatory because it has lost its solar power generator. Hatena switches identities like Dr. Jekyll and Mr. Hyde. The predatory alter-ego then grows a special feeding apparatus that allows it to acquire a new Nephroselmis algae from its environment, starting the endosymbiosis anew. Once the algae is acquired, the feeding apparatus is lost.
It is this amazing array of special adaptations toward a conjoined existence that makes the Hatena–Nephroselmis association such a wonderful example of an evolutionary transitional form probably undergoing selection toward permanent integration. It sheds light on one particular route that such evolution can take.
‘Kleptoplasty’ in Sea Slugs: Evolution by Purse-Snatching
Among animals that have gone the solar-powered way of plants, sea slugs in the group called Opisthobranchia have gone a step further even than Hatena in the reduction of the unneeded parts of their endosymbiotic algae [4]. Sea slugs normally use a rough, sand-paper-like row of teeth called a radula to scrape a meal of algae off the sea floor. However, the Eastern Emerald Elysia sea slug (Elysia chlorotica) sees the algae Vaucheria as more than a quick meal. It sees it for what matters most: its chloroplasts alone. But Vaucheria is a long, filamentous algae, with perhaps a little too much wrapping around the chloroplasts to practically hold onto the whole algae. No problem — Elysia simply performs a bit of purse snatching. It pierces the long cells of Vaucheria with its radula, and then sucks out the contents as if from a straw. It digests the contents of the Vaucheria cell, but stops short at the chloroplasts, which it claims for itself and maintains in its digestive tract cells in a functional state while the chloroplasts last.

The sea slug known as the Eastern Emerald Elysia (Elysia chlorotica), colored green by its kleptoplasts, stolen chloroplasts from the algae Vaucheria. (Image: Mary Paert)
This felony is called kleptoplasty, which literally means “stealing plastids” (a plastid is a membrane-bound organelle, such as a chloroplast). The chloroplasts lend Elysia a bright green color, hence its common name. Check out the original paper describing this interaction, “The making of a photosynthetic animal”, by Mary Rumpho and collaborators. Even if you do not read the technical parts of the manuscript, it’s very much worth a look to see the spectacular color photos of Elysia and other animals that use endosymbiotic algae for photosynthesis.
But the chloroplasts of Vaucheria are optimized for Vaucheria alone. Therefore, although they multiply inside cells of Vaucheria, the chloroplasts are unable to do so in the foreign environment of the sea slug’s cells, and they ultimately die. Still, they last for the entire 10-month life span of the sea slug, even though they are not transmitted to its offspring. How is this possible? Amazingly, studies have shown that Elysia has stolen not only chloroplasts from Vaucheria, but also some of its genes [4]! Some of the genes required for chloroplast maintenance are now found on the chromosomes of the sea slug, which greatly increases the ability of the sea slug to cultivate its stolen kleptoplasts. This is one of those rare examples of horizontal gene transfer between an animal and a microorganism. I covered horizontal gene transfer in more detail in a previous post, “Evolution’s Usual Suspects: 1. Plagiarizing Wizards“.
So Elysia is a photosynthetic animal that truly possesses chloroplasts, and even goes as far as to possess some of the genes necessary to maintain chloroplasts that cannot survive outside of their host. However, the association is not completely stable, for sufficient genes are not present for maintenance of reproducing populations of chloroplasts in its cells, so Elysia is not entirely photosynthetic.
An interesting side note about the stolen chloroplasts of Elysia: in a way, they are an example of tertiary endosymbiosis. The chloroplasts are highly modified red algae (with cyanobacterial chloroplasts of their own), which were in turn enslaved by Vaucheria, but were then enslaved yet again by Elysia. Poor red algae-derived chloroplasts — they are freed from one instance of metabolic slavery only to be enslaved afresh.
A Deep Sea Grand Theft Mystery Solved
Perhaps the most convoluted and colorful story of borrowed biological components hails from the deep sea, in the tale about the tiny but monstrous looking and equally intimidatingly-named Black Loose-jaw Dragonfish (Malacosteus niger). These bizarre creatures, like many other deep-sea fish, are bioluminescent, generating their own light through a biochemical reaction (more on this in a later post). They do this both to communicate with each other (e.g. find mates) and to illuminate their prey so that they can hunt more efficiently. Most of the light they generate is blue in color, and the eyes of deep sea animals are sensitive to such blue light. Bioluminescence in the deep sea is convenient, but it also therefore makes fish visible to their enemies, so it’s a risky superpower to possess.

The enigmatic Black Loose-jawed Dragonfish (Malacosteus niger), which installs light-sensitive pigments into its eyes that are derived from photosynthetic bacteria via copepods. (Image: Rafael Bañón)
However, dragonfish are unique among deep-sea fish in that they also generate red light, to which other fish — including their enemies — are blind, so they can escape their predators and more effectively illuminate their prey. But to be able to use red light, they must also be able to see it, and for that they have evolved a remarkable apparatus.
Their eyes contain a light-sensitive pigment that is not their own, but was stolen twice, once from a copepod (tiny plankton related to lobsters) and once from a photosynthetic bacterium [5]. (Recall that we can see light because the retinas of our eyes possess special compounds called pigments, which slightly change their molecular configuration when they are excited by light, and this excitation is transmitted to our nerves and ultimately our brains.) The special pigment in the eyes of dragonfish is derived from pigments called bacteriochlorophyll c and d. Bacteriochlorophyll c and d are actually photosynthetic pigments used to convert light to chemical energy (like plants use chlorophyll) by an ancient group of bacteria called green sulfur bacteria. Green sulfur bacteria are holdovers from a time over 2 billion years ago, when the earth’s atmosphere lacked free oxygen. So the pigment used by the fish to detect light is stolen from a bacterium that manufactures it to harness the energy in light.
But, the deep-sea dragonfish does not eat the bacteria directly. (You may think it obvious, for photosynthetic bacteria should not be found in the dark deep-sea, but green sulfur bacteria have in fact been isolated from hydrothermal vent communities in the deep-sea [6].) Instead, the dragonfish eat planktonic copepods, which in turn have eaten the bacteria and have retained the pigments in their bodies. This is like a thief stealing a stolen article from another thief. True, the dragonfish story does not quite qualify as endosymbiosis (no living components of another organism are harboured in the dragonfish’s cells), but it does serve to illustrate that the light-sensitive pigments of photosynthesis are valuable enough to encourage such grand theft between diverse creatures. Photosynthesis is one of the greatest inventions of life on earth, and I’ll cover more about it in a later post.
There are many, many other strange and awe-inspiring stories of endosymbiosis among earth’s living systems, but I will stop here to avoid writing a full-length book on the subject. Hopefully, however, this account has helped to inspire my readers to further investigate the ample trail of bread crumbs left by evolution in the form of endosymbioses.
References
1. J. Cameron Thrash, Alex Boyd, Megan J. Huggett, Jana Grote, Paul Carini, Ryan J. Yoder, Barbara Robbertse, Joseph W. Spatafora, Michael S. Rappé, Stephen J. Giovannoni. Phylogenomic evidence for a common ancestor of mitochondria and the SAR11 clade. Scientific Reports, 2011; 1 DOI: 10.1038/srep00013
2. Titlyanov EZ, Titlyanov TV, Leletkin VA, Tsukahara J, van Woesik R, Yamazato K. (1996) Degredation of zooxanthellae and regulation of their density in hermatypic corals. Marine Ecology Progress Series. 139: 167-178. (FREE DOWNLOAD)
3. Okamoto, Noriko; Inouye, Isao (October 2006). Hatena arenicola gen. et sp. nov., a katablepharid undergoing probable plastid acquisition. Protist 157(4): 401–19. doi:10.1016/j.protis.2006.05.011.
4. Rumpho M, Pelletreau KN, Moustafa A, Bhattacharya D. (2011) The making of a photosynthetic animal. The Journal of Experimental Biology 214:303-311 (FREE DOWNLOAD)
5. Douglas RH, Mullineaux CW, Partridge JC. (2000) Long-wave sensitivity in deep-sea stomiid dragonfish with far-red bioluminescence: evidence for a dietary origin of the chlorophyll-derived retinal photosensitizer of Malacosteus niger. Philosophical Transactions of the Royal Society of London, Series B. 355: 1269-1272. (FREE DOWNLOAD)
6. Beatty JT, Overmann J, Lince MT, Manske AK, Lang AS, Blankenship RE, Van Dover CL, Martinson TA, Plumley FG. (2005) An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent. Proceedings of the National Academy of Sciences of the United States of America 102 (26): 9306–10. doi:10.1073/pnas.0503674102 (FREE DOWNLOAD)